

Medical Devices
CELLONTECH is growing as the number one company in the bio-collagen industry.
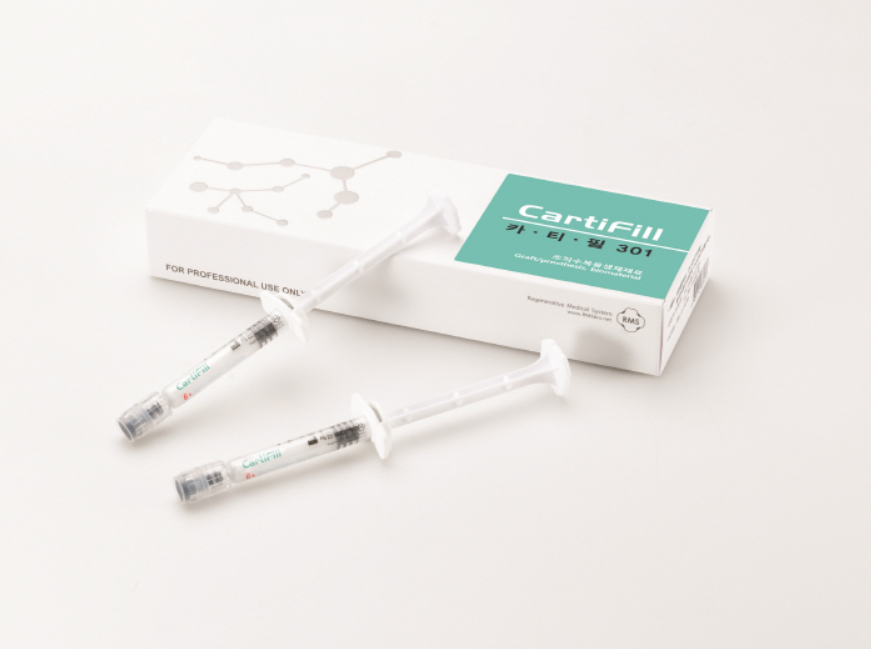
CartiFill
Effective in cartilage regeneration
through bone marrow blood
'Pure collagen' dissolved in a physiological phosphate buffer solution
Effective in cartilage regeneration through bone marrow blood, CartiFill, which consists of BioCollagen dissolved in a physiologically balanced phosphate buffer solution, is used to support the transplantation of autologous chondrocytes or bone marrow stimulation surgery in areas of articular cartilage defects. It is composed of BioCollagen, free from telopeptide regions where antigens are located, thereby minimizing immunological reactions.
• Provides a biophysical support matrix
• Secures stem cells in the injured area to promote cartilage regeneration
- - Leads to shortened treatment periods and regeneration into hyaline cartilage
• Simple application tailored to the defect area
- - Enabling minimally invasive surgery using arthroscopy
Applications
-
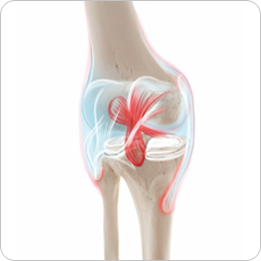
- · Cartilage defects
- · Knee joints
-
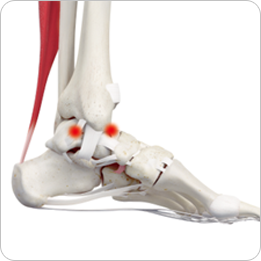
- · Cartilage defects
- · Ankle joints
Product Lineup
- · Product name
- Tissue Regeneration Supplement
- · Classification
- Medical device
- · Intended Use
- Used to support the transplantation of autologous chondrocytes or bone marrow stimulation surgery in areas of articular cartilage defects, aiding the positioning of chondrocytes and bone marrow
- · Model name
- CartiFill
- · Shelf life
- 36 months from the date of manufacture
- · Precautions
-
Prohibited for use in the following patients :
- - Patients with hypersensitivity to porcine proteins
- - Patients with hypersensitivity to implants
- - Patients with known hypersensitivity conditions
- - Patients with infectious diseases
- - Patients or family members with a history of autoimmune diseases or previous hypersensitivity reactions to the components of this medical device
- - Patients with contraindications specified for fibrin sealant usage
(including patients with a hypersensitivity to aprotinin, severe arterial sclerosis, or venous bleeding as indicated in the contraindications section of the fibrin sealant product).